


(25 February 2026)
 Paper published on Lancet Digital Health RareArena - a comprehensive benchmark dataset unveiling the potential of large language models in rare disease diagnosis at 10.1016/j.landig.2025.100953
Paper published on Lancet Digital Health RareArena - a comprehensive benchmark dataset unveiling the potential of large language models in rare disease diagnosis at 10.1016/j.landig.2025.100953
The study introduces RareArena, a new large benchmark dataset designed to improve how artificial intelligence (AI), especially large language models (LLMs), is evaluated for rare disease diagnosis. Medical AI has shown promise in interpreting clinical text and supporting diagnosis, but existing evaluations often rely on small or artificial test sets that don’t reflect the real complexity doctors face. RareArena tackles this gap by collecting thousands of real clinical case reports from PubMed Central and organising them into tasks that mimic real-world clinical problem-solving — such as screening for rare diseases and confirming specific diagnoses from free-text descriptions. The dataset enables systematic comparison of AI models’ abilities to understand medical language and recognise rare conditions, highlighting strengths and limitations in current systems. By providing a realistic testing ground, RareArena aims to accelerate the development of safer, more trustworthy AI tools that can support clinicians in identifying rare diseases earlier and more accurately.
Read it at 10.1016/j.landig.2025.100953.
(3 February 2026)
 Paper published on Radiology Guidelines for Reporting Studies on Large Language Models in Radiology - An International Delphi Expert Survey at https://pubs.rsna.org/doi/full/10.1148/radiol.250913
Paper published on Radiology Guidelines for Reporting Studies on Large Language Models in Radiology - An International Delphi Expert Survey at https://pubs.rsna.org/doi/full/10.1148/radiol.250913
Large language models (LLMs) have transformative potential in radiology, including textual summaries, diagnostic decision support, proofreading, and image analysis. However, the rapid increase in studies investigating these models, along with the lack of standardized LLM-specific reporting practices, affects reproducibility, reliability, and clinical applicability. To address this, reporting guidelines for LLM studies in radiology were developed using a two-step process. First, a systematic review of LLM studies in radiology was conducted across PubMed, IEEE Xplore, and the ACM Digital Library, covering publications between May 2023 and March 2024. Of 511 screened studies, 57 were included to identify relevant aspects for the guidelines. Then, in a Delphi process, 20 international experts developed the final list of items for inclusion. Items consented as relevant were summarized into a structured checklist containing 32 items across six key categories - general information and data input; prompting and fine-tuning; performance metrics; ethics and data transparency; implementation, risks, and limitations; and further/optional aspects. The final FLAIR (Framework for LLM Assessment in Radiology) checklist aims to standardize reporting of LLM studies in radiology, fostering transparency, reproducibility, comparability, and clinical applicability to enhance clinical translation and patient care..
Read it at https://pubs.rsna.org/doi/full/10.1148/radiol.250913.
(16 January 2026)
 Preprint Prevalence of 406 rare diseases by ethnicity and their associated COVID-19 infection burden - A national cross-sectional study of 62.5 million people in England at https://www.medrxiv.org/content/10.64898/2026.01.13.26344068v1
Preprint Prevalence of 406 rare diseases by ethnicity and their associated COVID-19 infection burden - A national cross-sectional study of 62.5 million people in England at https://www.medrxiv.org/content/10.64898/2026.01.13.26344068v1
The burden of the COVID-19 pandemic disproportionately affected individuals with rare diseases. However, the patterning of this risk by ethnicity is complex and runs contrary to general population trends, likely reflecting the deep-seated ethnic disparities in the prevalence of specific RDs. Our foundational map of 406 rare diseases by granular ethnicity is essential for understanding these factors and identifying which specific patient-ethnic subgroups face the greatest intersectional risk.
Read the preprint version at https://www.medrxiv.org/content/10.64898/2026.01.13.26344068v1.
(4 November 2025)
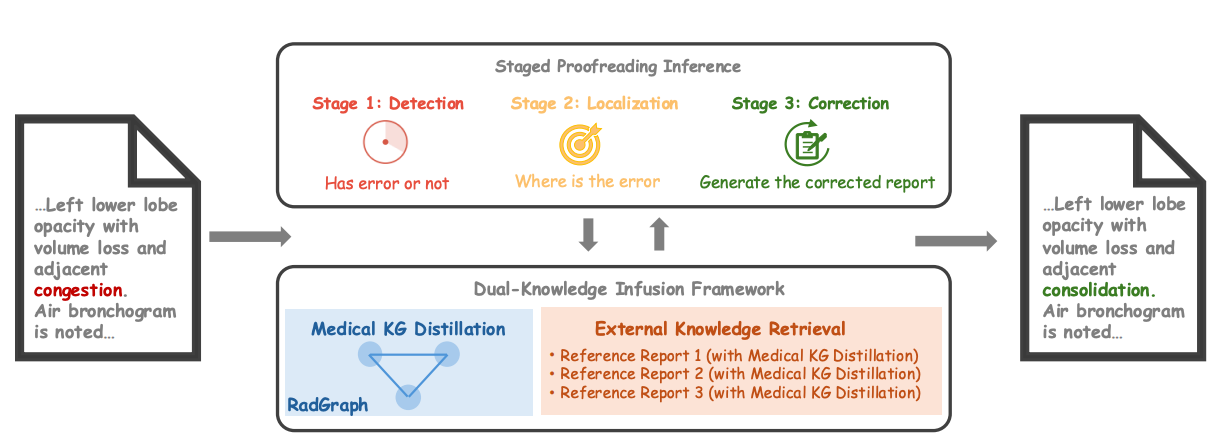 Paper accepted by AAAI 2026 Artificial Intelligence for Social Impact Track – Error Correction in Radiology Reports - A Knowledge Distillation-Based Multi-Stage Framework at https://arxiv.org/abs/2406.15045v3
Paper accepted by AAAI 2026 Artificial Intelligence for Social Impact Track – Error Correction in Radiology Reports - A Knowledge Distillation-Based Multi-Stage Framework at https://arxiv.org/abs/2406.15045v3
The increasing complexity and workload of clinical radiology leads to inevitable oversights and mistakes in their use as diagnostic tools, causing delayed treatments and sometimes life-threatening harms to patients. While large language models (LLMs) have shown remarkable progress in many tasks, their utilities in detecting and correcting errors in radiology reporting are limited. We present a novel dual-knowledge infusion framework that enhances LLMs’ capability for radiology report proofreading through systematic integration of medical expertise. Specifically, our knowledge infusion combines medical knowledge graph distillation (MKGD) with external knowledge retrieval (EXKR), enabling an effective automated approach in tackling mistakes in radiology reporting. By decomposing the complex proofreading task into three specialized stages of detection, localization, and correction, our method mirrors the systematic review process employed by expert radiologists, ensuring both precision and clinical interpretability. The dual-knowledge framework captures intricate medical relationships through structured graph representations while leveraging curated clinical expertise from reference reports. To perform a robust, clinically relevant evaluation, we constructed a comprehensive benchmark using real-world radiology reports with error patterns derived from real-world scenarios, including speech recognition confusions, terminology ambiguities, and template-related inconsistencies, all validated by practicing radiologists. Extensive evaluations across multiple LLM architectures demonstrate substantial improvements of our approach - up to 31.56% increase in error detection accuracy and 37.4% reduction in processing time. Human evaluation by radiologists confirms superior clinical relevance and factual consistency compared to existing approaches. Our framework addresses critical needs in clinical practice by enhancing report quality while reducing radiologist burden, particularly benefiting resource-constrained healthcare environments.
Read the preprint version at https://arxiv.org/abs/2406.15045v3.
(31 October 2025)
 A systematic review published on Communications Medicine - Digital health technology use in clinical trials of rare diseases - a systematic review at https://www.nature.com/articles/s43856-025-01137-6
A systematic review published on Communications Medicine - Digital health technology use in clinical trials of rare diseases - a systematic review at https://www.nature.com/articles/s43856-025-01137-6
Hundreds of millions of people around the world live with rare diseases, but most of these conditions still don’t have effective treatments. Clinical trials are vital for new therapies, but rare disease trials are especially challenging due to small, dispersed patient populations and difficulties with long-term participation. This study looked at how digital health tools—like wearable devices, mobile apps, and healthcare platforms—are being used to improve rare disease clinical trials. We analysed 262 trials across ten rare diseases and found that digital health technologies were increasingly used for remote monitoring, digital treatment, and long-term care. By making it easier for patients to participate in trials—no matter where they live—digital technologies can help speed up the development of much-needed treatments and help make research more inclusive and equitable by reaching patients who would otherwise be left out.
Read the full article at https://www.nature.com/articles/s43856-025-01137-6.
(21 October 2025)
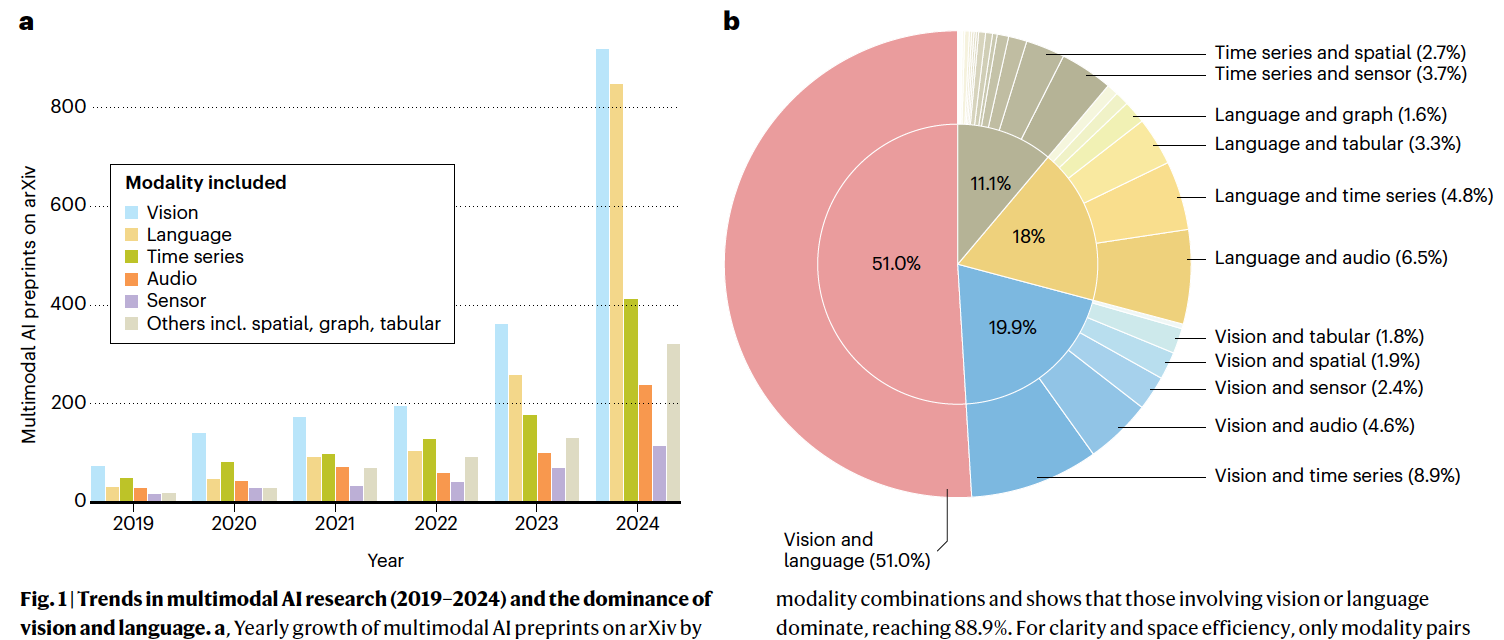 A collaborative, perspective paper published on Nature Machine Intelligence - Towards deployment-centric multimodal AI beyond vision and languag at https://www.nature.com/articles/s42256-025-01116-5
A collaborative, perspective paper published on Nature Machine Intelligence - Towards deployment-centric multimodal AI beyond vision and languag at https://www.nature.com/articles/s42256-025-01116-5
Multimodal artificial intelligence (AI) integrates diverse types of data via machine learning to improve understanding, prediction and decision-making across disciplines such as healthcare, science and engineering. However, most multimodal AI advances focus on models for vision and language data, and their deployability remains a key challenge. We advocate a deployment-centric workflow that incorporates deployment constraints early on to reduce the likelihood of undeployable solutions, complementing data-centric and model-centric approaches. We also emphasize deeper integration across multiple levels of multimodality through stakeholder engagement and interdisciplinary collaboration to broaden the research scope beyond vision and language. To facilitate this approach, we identify common multimodal-AI-specific challenges shared across disciplines and examine three real-world use cases - pandemic response, self-driving car design and climate change adaptation, drawing expertise from healthcare, social science, engineering, science, sustainability and finance. By fostering interdisciplinary dialogue and open research practices, our community can accelerate deployment-centric development for broad societal impact.
Read the full article at https://www.nature.com/articles/s42256-025-01116-5.
(19 September 2025)
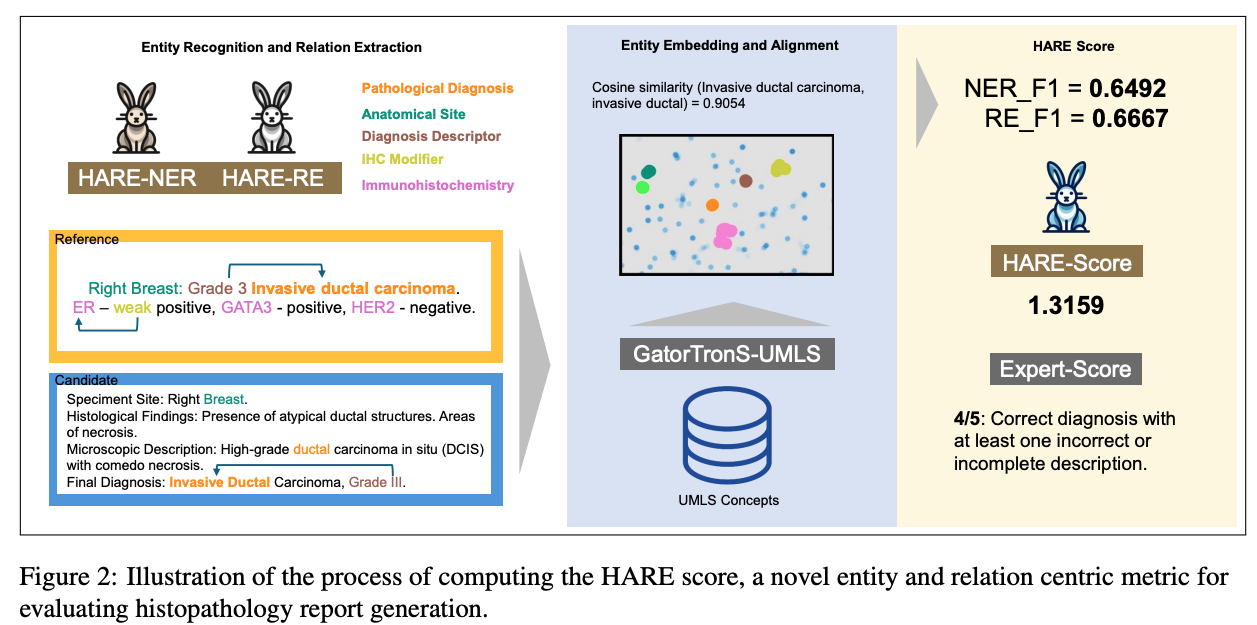 Paper accepted by EMNLP 2025 findings – HARE- an entity and relation centric evaluation framework for histopathology reports – at https://arxiv.org/abs/2507.09097
Paper accepted by EMNLP 2025 findings – HARE- an entity and relation centric evaluation framework for histopathology reports – at https://arxiv.org/abs/2507.09097
Large Vision-Language Models (LVLMs) have demonstrated promising performance in chest X-ray (CXR) analysis. To enhance human-computer interaction, several studies have incorporated radiologists’ eye gaze, typically through heatmaps or textual prompts. However, these methods often overlook the sequential order of eye movements, which could provide valuable insights by highlighting both the areas of interest and the order in which they are examined. In this work, we propose a novel approach called RadEyeVideo that integrates radiologists’ eye-fixation data as a video sequence, capturing both the temporal and spatial dynamics of their gaze. We evaluate this method in CXR report generation and disease diagnosis using three general-domain, open-source LVLMs with video input capabilities. When prompted with eye-gaze videos, model performance improves by up to 24.6% in the report generation task and on average 15.2% for both tasks using scaled evaluation metrics. Notably, RadEyeVideo enhanced an open-domain LVLM model, LLaVA-OneVision, to surpass task-specific medical LVLMs such as MAIRA-2 and CheXagent, trained on large Chest X-ray data. This work highlights that domain expert’s knowledge (eye-gaze information in this case), when effectively integrated with LVLMs, can significantly enhance general-domain models’ capabilities in clinical tasks. RadEyeVideo is a step toward a scalable human-centered approach of utilizing LVLMs in medical image analytics.
Read the full paper at https://arxiv.org/abs/2507.09097.
(8 August 2025)
 Tokenizer work published - Infusing clinical knowledge into language models by subword optimisation and embedding initialisation - now with Computers in Biology and Medicine.
Tokenizer work published - Infusing clinical knowledge into language models by subword optimisation and embedding initialisation - now with Computers in Biology and Medicine.
This study introduces a novel knowledge enhanced tokenisation mechanism, K-Tokeniser, for clinical text processing. Technically, at The study proposes a novel tokenisation method utilising global representations of tokens based on domain-specific concepts (e.g., drugs, diseases) from ontologies like UMLS or task-specific corpora. At training or inference, word and sentence-level optimisation is used to select the optimal token representations. It proposes an embedding initialisation approach for new tokens, eliminating the need for pre-training the language models. The Model built using K-Tokeniser achieves a notable 13% increase in Micro F1 score for automated clinical coding. It requires only 50% of training data for concept extraction and less than 20% for automated coding to outperform the baseline clinicalBERT model.
Read it at 10.1016/j.compbiomed.2025.110747.
(28 May 2025)
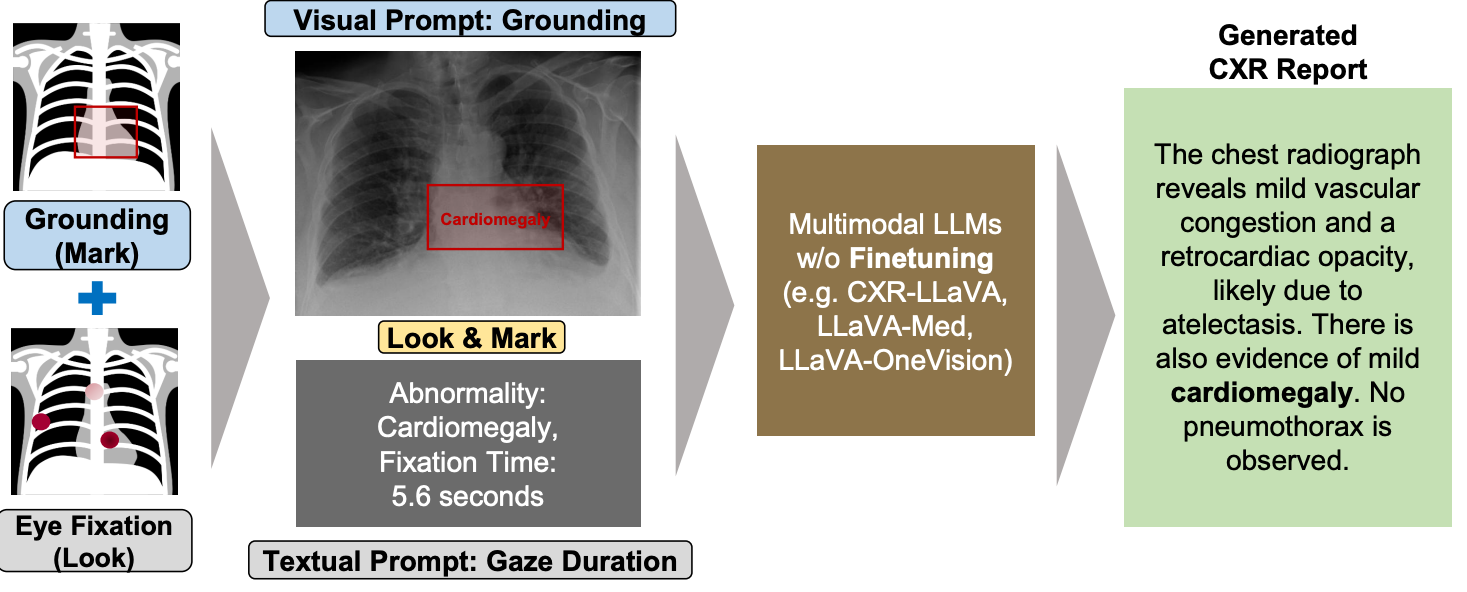 Paper accepted by ACL 2025 findings – Look & Mark - Leveraging Radiologist Eye Fixations and Bounding boxes in Multimodal Large Language Models for Chest X-ray Report Generation – at https://arxiv.org/abs/2505.22222
Paper accepted by ACL 2025 findings – Look & Mark - Leveraging Radiologist Eye Fixations and Bounding boxes in Multimodal Large Language Models for Chest X-ray Report Generation – at https://arxiv.org/abs/2505.22222
This study introduces Look & Mark (L&M), a novel approach to radiology report generation that integrates radiologist fixation cues (Look) with bounding box annotations (Mark) to guide multimodal Large Language Models (LLMs). By combining these complementary grounding strategies, L&M significantly improves the clinical relevance of generated reports, reduces hallucinations, and enhances model alignment with real-world diagnostic workflows. Importantly, L&M achieves these gains without requiring extensive fine-tuning, leveraging in-context learning to adapt both general-purpose and domain-specific models alike.
Our experiments demonstrate that L&M significantly boosts performance across both lexical and clinical evaluation metrics, with the largest gains observed in clinical metrics such as RaTEScore and RadGraph-XL. For instance, CXR-LLaVA achieved a 1.2% improvement in overall metrics (A.AVG) compared to baseline prompting, while LLaVA-Med demonstrated a remarkable 9.2% boost. General-purpose models also benefited significantly, with LLaVA-OV achieving an 87.3% clinical average (C.AVG), the highest among all tested models, even surpassing domain-specific models trained explicitly for chest X-ray report generation.
Read the full paper at https://arxiv.org/abs/2505.22222.
(28 May 2025)
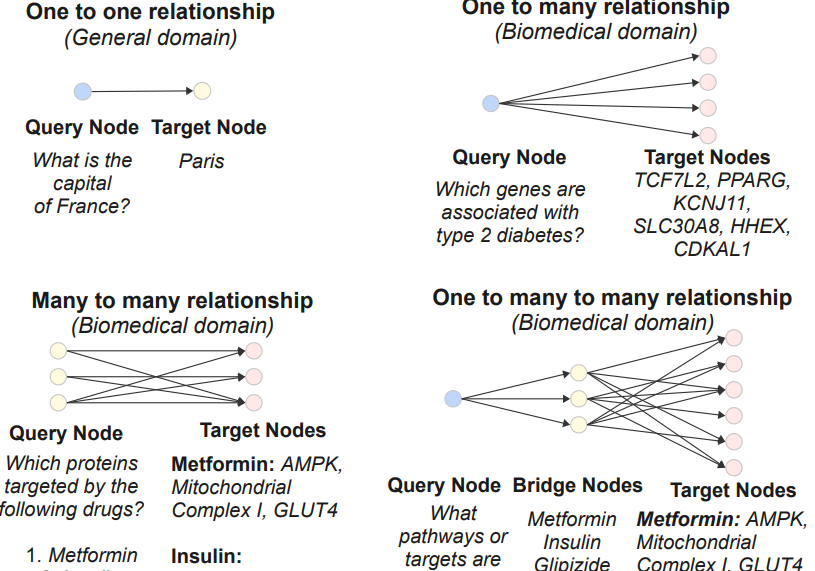 Paper accepted by ACL 2025 findings – BioHopR - A Benchmark for Multi-Hop, Multi-Answer Reasoning in Biomedicine – at https://arxiv.org/abs/2505.22240
Paper accepted by ACL 2025 findings – BioHopR - A Benchmark for Multi-Hop, Multi-Answer Reasoning in Biomedicine – at https://arxiv.org/abs/2505.22240
This paper introduces BioHopR, a benchmark for evaluating multi-hop, multi-answer reasoning in the biomedical domain. Built on the PrimeKG knowledge graph, BioHopR captures the complexity of real-world biomedical queries through one-tomany and many-to-many relationships, rigorously assessing reasoning over 1-hop and 2-hop tasks. Evaluation results highlight that O3-mini, a proprietary model with a reasoning step, outperforms open-source models including biomedical models like HuatuoGPT-o1. Across all models, the performance drop from 1-hop to 2-hop tasks underscores the difficulty of aligning intermediate reasoning steps, especially in bridging entities.
Read the full paper at https://arxiv.org/abs/2505.22240.
(12 May 2025)
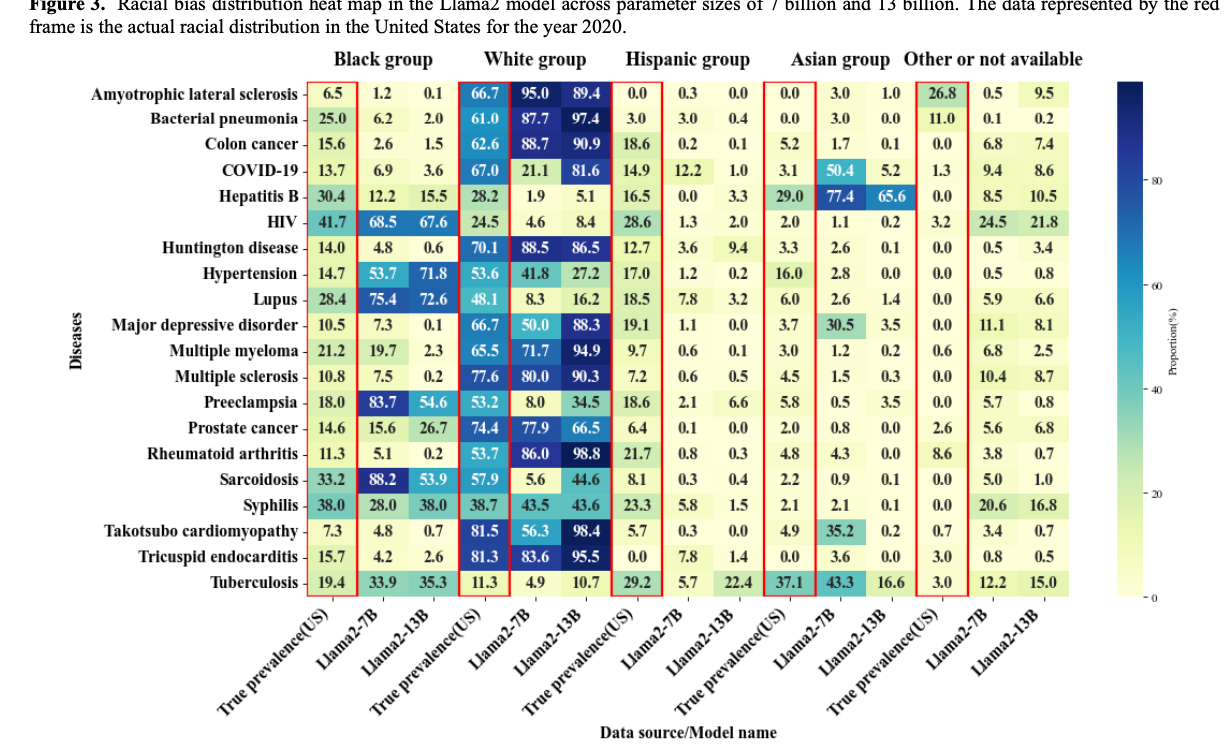 Paper accepted by Journal of Medical Internet Research – Evaluation and Bias Analysis of Large Language Models in Generating Synthetic Electronic Health Records - Comparative Study – at https://doi.org/10.2196/65317
Paper accepted by Journal of Medical Internet Research – Evaluation and Bias Analysis of Large Language Models in Generating Synthetic Electronic Health Records - Comparative Study – at https://doi.org/10.2196/65317
Larger models, such as Yi-34B, Qwen-14B, and Llama 2 to 13 B, showed improved performance in generating more comprehensive EHRs, as reflected in higher EPS values. However, this increased performance was accompanied by a notable escalation in both gender and racial biases, highlighting a performance-bias trade-off. The study identified 4 key findings as follows (1) as model size increased, EHR generation improved, but demographic biases also became more pronounced; (2) biases were observed across all models, not just the larger ones; (3) gender bias closely aligned with real-world disease prevalence, while racial bias was evident in only a subset of diseases; and (4) racial biases varied, with some diseases showing overrepresentation of White or Black populations and underrepresentation of Hispanic and Asian groups. These findings underline the need for effective bias mitigation strategies and the development of benchmarks to ensure fairness in artificial intelligence applications for health care.
Read the full paper at https://doi.org/10.2196/65317.
(18 April 2025)
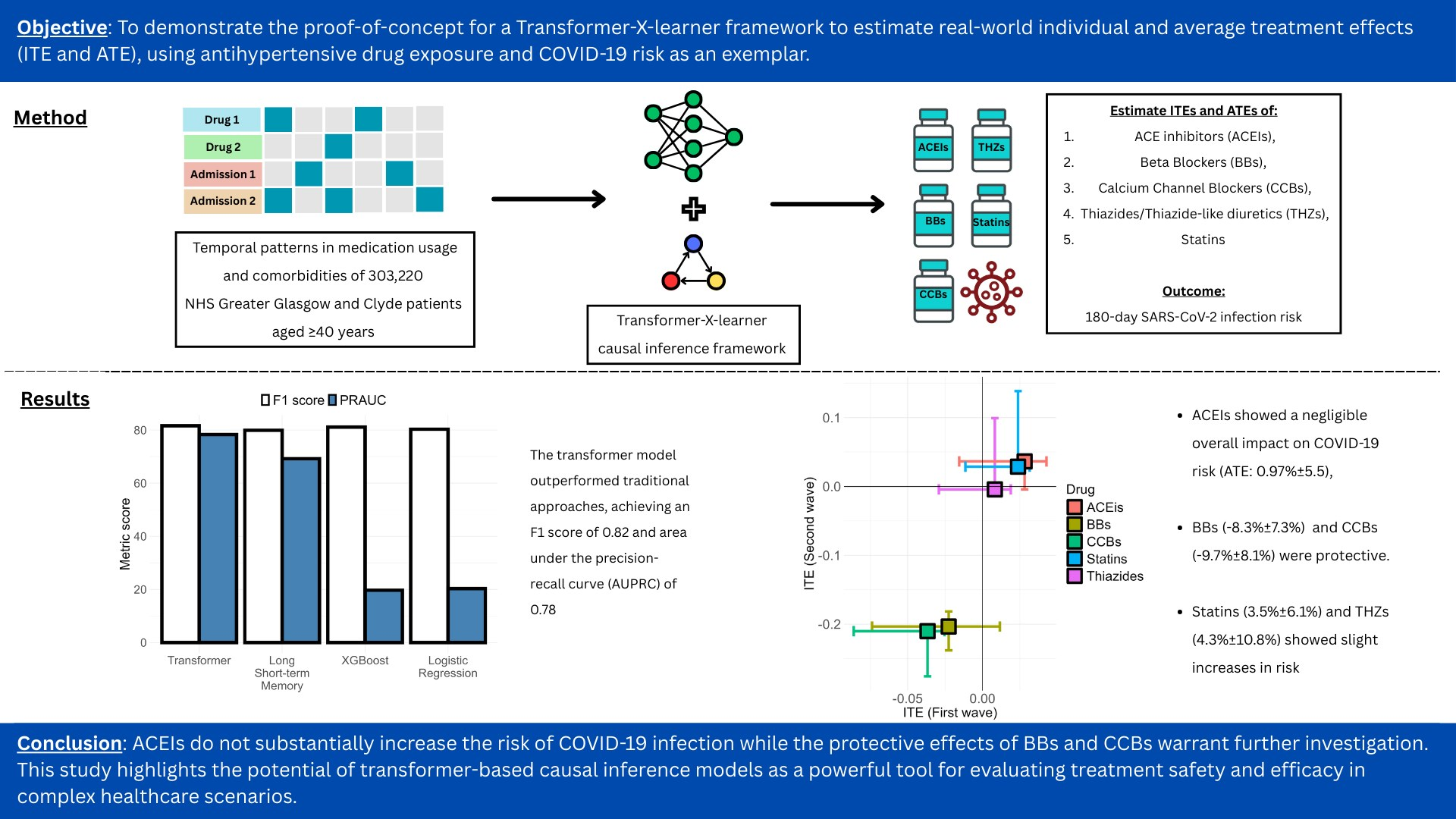 Published in American Journal of Hypertension – A Transformer-Based Framework for Counterfactual Estimation of Antihypertensive Treatment Effect on COVID-19 Infection Risk - A Proof-of-Concept Study – at https://doi.org/10.1093/ajh/hpaf055
Published in American Journal of Hypertension – A Transformer-Based Framework for Counterfactual Estimation of Antihypertensive Treatment Effect on COVID-19 Infection Risk - A Proof-of-Concept Study – at https://doi.org/10.1093/ajh/hpaf055
A new study in the American Journal of Hypertension investigates the relationship between antihypertensive medications and COVID-19 infection risk. The research employed a transformer-based framework to analyze real-world data from over 300,000 patients.
Key findings indicate that while ACE inhibitors showed a negligible effect on COVID-19 risk, beta-blockers and calcium channel blockers were associated with a protective effect. Statins and thiazides showed a slight increase in risk.
This study demonstrates the potential of advanced causal inference models in evaluating treatment outcomes in complex healthcare scenarios and offers important insights for clinical consideration.
Read the full paper at https://doi.org/10.1093/ajh/hpaf055.